Feb 17 2021
元素周期表 - 有助于识别和估计其性质趋势的要素的安排 - 向所有化学专业的学生讲授。例如,科幻小说的作者偶尔会根据硅元素来解释生命,因亚博老虎机网登录为它与碳的周期表中的同一列出现在同一列中。
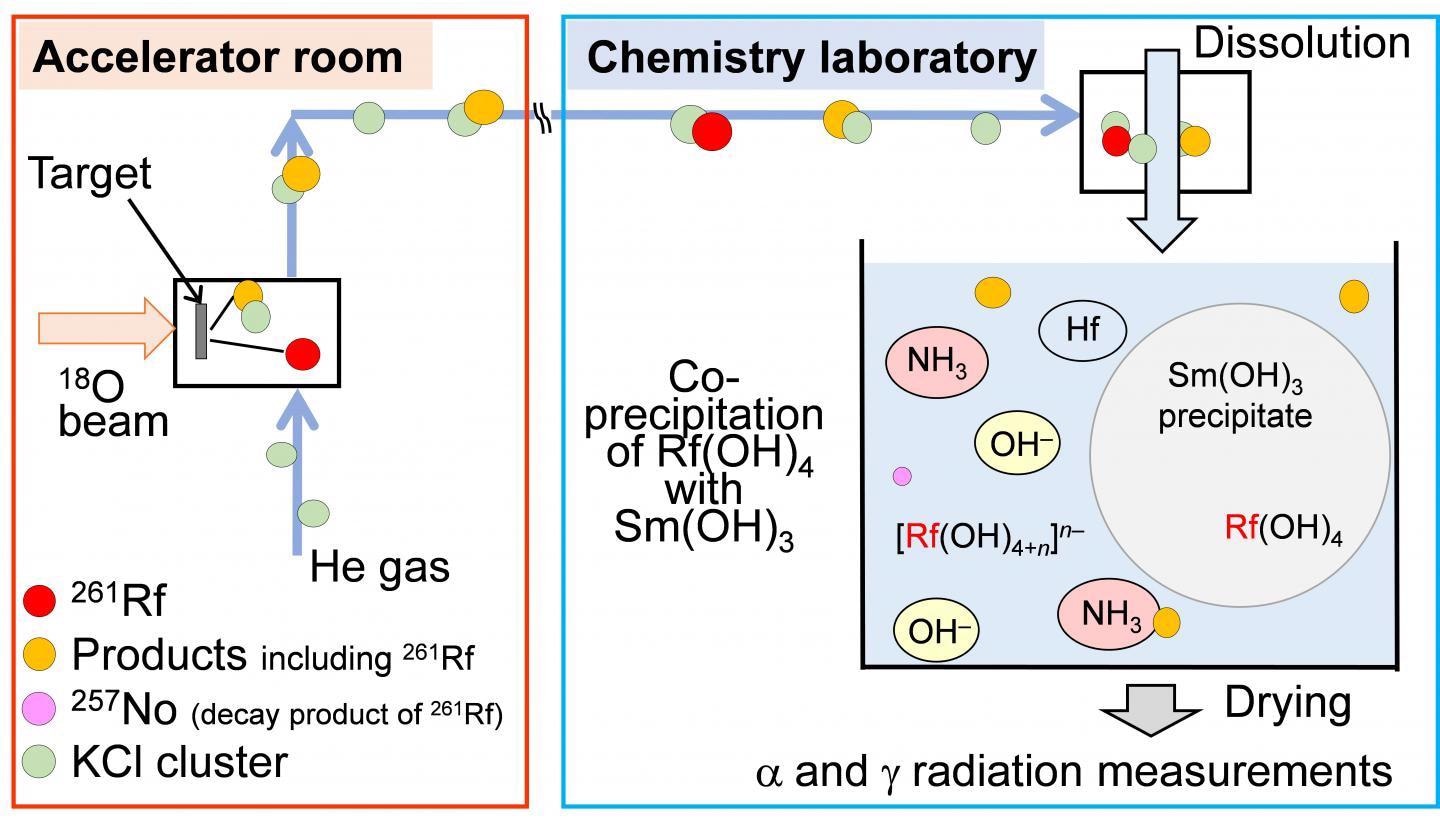 在线共同沉积实验的示意图261Rf. Image Credit: Osaka University.
在线共同沉积实验的示意图261Rf. Image Credit: Osaka University.
但是与周期表的预期趋势有些偏差。例如,在周期表的同一列中发现了锡和铅,因此,应具有几乎相同的特征。但是,尽管经常在汽车中使用铅酸电池,但锡酸电池的目的却没有相同的目的。
Today, it is well known that the reason behind this aspect is that a large part of the energy in lead-acid batteries is attributed to relativistic chemistry; however; such chemistry was not known to the scientists who initially suggested the periodic table.
It is hard to study relativistic chemistry in superheavy elements. This is because these elements are usually created one at a time in nuclear fission reactions and they tend to decompose rapidly.
尽管如此,分析超重量元素的化学的潜力也许可以揭示针对超重量元素和标准较轻元素(例如黄金和铅)的新颖应用。
In a new study published in the自然化学杂志,大阪大学researchers investigated the reactions between single atoms of superheavy rutherfordium metal and two groups of common bases. Experiments like these will allow scientists to use relativistic principles to better use the chemistry of several elements.
我们准备了Riken Accelerator研究设施的Rutherfordium的单个原子,并试图用氢氧化原子或胺基碱反应这些原子。Radioactivity measurements indicated the end result。
Yoshitaka Kasamatsu, Study Lead Author, Osaka University
Such experiments will help scientists gain a better understanding of relativistic chemistry. For instance, rutherfordium can create precipitate compounds with hydroxide base at all concentrations of the base, but at high concentrations, it forms its homologs, hafnium and zirconium. This variation in reactivity could be attributed to relativistic chemistry.
If we had a way to produce a pure rutherfordium precipitate in larger quantities, we could move forward with proposing practical applications. In the meantime, our studies will help researchers systematically explore the chemistry of superheavy elements。
Atsushi Shinohara, Study Senior Author, Osaka University
Relativistic chemistry describes why bulk gold metal is not colored in silver, as one would anticipate based on the predictions of the periodic table. This chemistry also describes why mercury metal remains in a liquid state at room temperatures, in spite of the predictions of the periodic table.
There may be several unforeseen applications that emerge from learning about the chemistry of superheavy elements. Such discoveries will rely on recently reported procedures as well as ongoing fundamental analyses, like the one performed by the research team from Osaka University.
期刊参考
Kasamatsu, Y.,等。(2021)。基本解决方案中卢瑟福族的单个原子的共沉淀行为。自然化学。doi.org/10.1038/s41557-020-00634-6。
来源:https://www.osaka-u.ac.jp/en