Titration is an analytical method frequently used within the industry. Manual, semi-automated, and fully automated titrations are popular options and are discussed in-depth in many academic studies.
在将其与手动滴定进行比较时,已经在自动滴定中确定了一些好处和缺点,尤其是测量精度的提高以及可以制定的大量时间和成本储蓄。
滴定如何开始
Titration是一种主要的分析方法,以非常易于完成而闻名。唯一必需的项目是架,滴定器和适当的端点指示器,因此这也不是最古老的定量分析方法之一。
在18th世纪,弗朗索瓦·安托万·亨利·萨克里西尔斯1发明了第一个架。这一过程是由卡尔·弗里德里希·莫尔(Karl Friedrich Mohr)进一步开发的,他于1855年撰写了有关滴定的第一本书,称为“分析化学中的滴定方法教学簿”。
滴定原则
1811年2,Amedeo Avogadro首先建议在标准条件下,无论其包含哪种类型的分子,一定体积的气体中的分子数总是保持不变。后来,在1909年,让·佩林(Jean Perrin3。
该定义解释说,定义的特定物质质量包含精确数量的分子。因此,还定义了溶液中分子的数量。从此领先,滴定诞生了。它被认为与计算样品基质中物质(分析物)的分子一样简单。
The volume of titrant is used to enable a chemical reaction with the analyte, and titration determines this. All chemical reactions occur in distinct stoichiometric ratios, and so the analyte concentration can be determined.
This is done by identifying the precise level of concentration of the titrant and calculating its consumption, or volume, using a measurable endpoint. Manual titration using a typical benchtop setup can be seen in Figure 1.
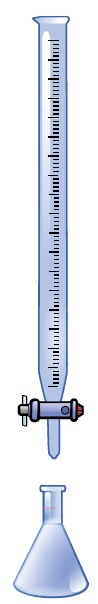
Figure 1.A common setup for a manual titration where the buret is filled with titrant, and the Erlenmeyer flask consists of the sample solution, which contains the analyte that needs to be measured.
The Ability of Titration Indicators to Determine pH
pH滴定在其他几种滴定变化中最常见。端点处的pH值可能由于酸解离常数而有所不同(PKa) varying depending on each separate acid. A selection of pH indicators that cover most of the pH range from 0–14 is shown in Figure 2.
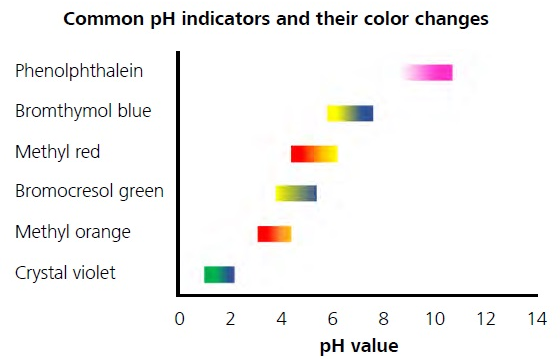
图2。Color variations of different pH indicators depending on the pH value.
The color change of an appropriate endpoint can also be used to determine the endpoint of additional titration types, such as redox, complexometric, or argentometric titrations. Table 1 lists examples of the indicators used for these different reaction types.
表格1。Endpoint indicators frequently used for different reaction types.
| 滴定类型 |
Indicator |
| 复合法 |
eriiochrome黑色T MUREXIDE |
| Argentric |
Potassium dichromate
铁(III)硫酸铵 |
| Redox |
Change of color from titrant (e.g., potassium permanganate) Starch solution |
For each of these individual indicators, the color change is hard to predict accurately, particularly for the complexometric reactions. The color change depends on the pH value and the complex metal equally.
手动滴定挑战
即使手动滴定已有近200年的历史,但仍经常使用和引用许多标准和规范。但是,手动滴定面临一些并发症。
Visual Perception
The main reason for these challenges is the visual perception of the endpoint. Each individual experiences colors and color intensity differently, which results in deviations in the manual titration depending on the specific analyst performing the task and their bias. Figure 3 exemplifies this idea and the challenge in deciding upon an endpoint visually.

图3。用C(NaOH)= 1 mol/L和苯甲胺蛋白作为指示剂的C(HCl)= 1 mol/L的滴定。The individual images vary only when adding a single drop of the NaOH titrant。
在每种情况下,在图3中1-5中获得的颜色强度仅在大约50 µL NaOH滴定剂中变化。问的问题是应在哪里选择“正确”的端点。如果每位分析师以完全相同的方式处理,测量的准确性将受到影响。
墨滴大小
The results of manual titration can only be as precise as the smallest drop size from the buret. One drop is defined to be 50 µL within the pharmaceutical industry, which means that a maximum precision regarding a 50 µL drop size can be attained. Assuming a consumption of about 5 mL titrant, this can lead to a 1% error or more.
Buret Error
如所有玻璃器皿所见,该柜员本身具有特殊的容忍度。允许50毫升架的公差为50 µl。但是,其他错误源可能是由处理架(例如视差错误)引起的。如果用户没有水平查看半月板,而是从角度看,则可能发生此错误。半月板读数取决于视角,如图4所示。
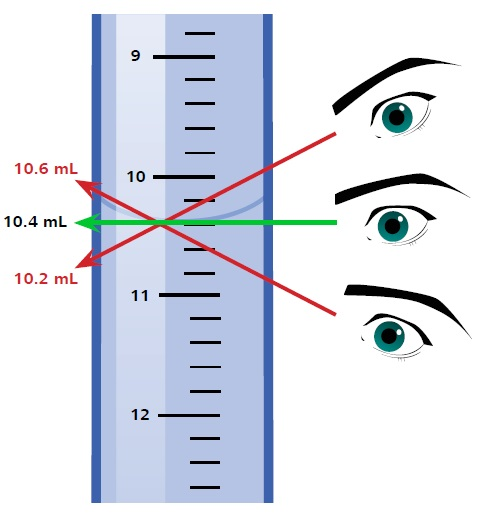
图4。半月板读数取决于观看角,导致视差错误。
The points discussed demonstrate that although it is easy to perform manual titration, results are largely influenced by the user. Additionally, manual titration does not provide cost-effective benefits due to the extended length of time required for cleaning, refilling the buret, and manually calculating the results.
此外,关于实验室人员存在安全性问题,因为在室内补充装置期间很容易溢出化学药品。数据完整性是手动滴定的另一个考虑因素,因为所有数据都被手动传输到笔记本或计算机中。没有进行自动计算,增加了与人为错误有关的问题,这是这种情况下的潜在风险。
半自动滴定的开发
为了解决手动滴定精度和精度不足的问题,可以使用电子架。它涉及使用电动机驱动的主轴和充满滴定剂的玻璃缸。
最近几代设备带有添加的内置搅拌器,并让用户完成自动计app亚博体育算,并将结果保存在存储设备(例如USB棒或PC)上,或者让他们在分析后立即打印结果。
半自动滴定的精度
When converting from manual procedures to semi-automated titration, the primary enhancements are with regards to the accuracy and precision. Electronic burets are able to dose as precisely as 2.5 µL for a 50 mL cylinder unit, which improves the accuracy by a factor of 20 in comparison to a manual titration.
However, a large drawback that persists is the subjectivity of visual perception. Subsequently, the next stage in titration evolution is automated potentiometric titration. In the mid-1960s, automated potentiometric titration was developed and overcame many of the remaining disadvantages associated with the process.
Data collection, equivalence point determination, and evaluation can be done automatically, which displays enormous benefits:
- Accuracy and precision improvements
- 耗时手动流程的时间更少
- 人为错误潜力减少
更好的准确性和精度
With modern auto-titrators, a resolution of 10,000–100,000 steps can be reached, corresponding to a precision of 5 µL decreased to 0.5 µL for a 50 mL motor-driven buret. The precision can be further improved by utilizing a motor-driven buret with a smaller volume.
Visual Perception Concerns Removed
Without the development of sensors, it wouldn’t be possible to establish automated titration. The first glass electrode for potentiometric titration was manufactured in 1909. Potentiometric sensors give an endpoint or equivalence point determination separate from color change or bias from a user.
由于许多样品需要不同的电极属性,因此现在有大量传感器可以访问它。表2列出了不同类型的滴定类型的示例。
Optrode是一个传感器,在研究自动滴定时必须具体提及。仍然经常使用带有颜色指示的滴定,因此开发开始创建Optrode。
它是一个传感器,可以通过定义特定波长的吸光度变化来识别微小的颜色差异。然后,它将吸收转换为可以测量的电位。以这种方式,比色滴定也会变得越来越准确和精确。精度提高是因为结果不再取决于实验室使用者的视觉感知。
Traceable Results for Data Integrity
Manual titration methods involve all results being read from the buret and documented in the lab journal or typed manually into the software. This process poses error risk, with an increased likelihood of incorrect value transfer.
In order to solve the problem of incorrect value transfer, automated titrations document the measured values into a measuring point list, and automatic calculation of results is performed on the device. These results can be printed with the date and time displayed, or exported as a PDF file.
所需的样本较少
通过手动滴定,足够的准确性和精度值需要大量样品来获得特定范围内的滴定消耗,并解决了量增加的量的误差,该误差可能高达0.05 mL。
表2。Specific electrodes for a variety of applications.
| Sensor |
Application |
 |
通用实验室使用,pH 0-14 |
 |
水,一般,pH 0-13 |
 |
For redox titrations without any change in pH value |
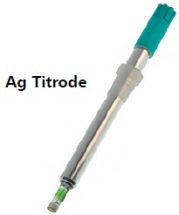 |
对于无需任何变化的pH值滴定 |
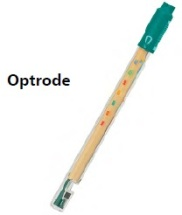 |
For titrations with indicators and a color change |
Conversely, the volume addition by a motor-driven buret might decrease to 0.5 µL, allowing the operator to lessen the sample size and to use a reduced amount of chemical reagents. It is suggested to utilize between 10–90% of the cylinder volume of the buret and to alter the sample size accordingly.
具有成本效益的滴定
The highest cost of any manual titration is the cost of labor. Laboratory analysts need to be suitably trained and, during titration, are fully occupied. With automated titrations, on the other hand, this completely changes. The procedure on the autotitrator is programmed one time, and can then be recalled whenever needed by any member of personnel simply by pushing a button.
Manual and automatic analysis times required for a series of five titrations were compared. A summary of the results is shown in Table 3.
Table 3.五个确定为10 mL C(NaOH)= 0.1 mol/L的摘要,C(HCl)= 0.1 mol/l。所有滴定均由相同的解决方案和移液器完成。
|
自动滴定 |
Manual titration* |
| Mean value |
10.0023毫升 |
9.92 mL |
| 标准开发abs. |
0.009 mL |
0.04毫升 |
| 标准开发rel。 |
0.09% |
0.4% |
| Total duration |
32min 33 s |
14分钟53 S |
Duration with needed
presence of analyst |
1分钟33 S |
14分钟53 S |
*对于手动滴定,将苯甲胺蛋白用作指标。
This case displays that manual titration is quicker than the自动滴定。但是,结果准确得多。在6分12 s时测量每个自动滴定的平均分析时间,这意味着在五个确定的系列中,实验室人员的存在仅占每个分析约1分钟33 s。
In comparison, during manual titration, the lab personnel is required throughout the whole titration, accounting for a significant amount of time saved.
因此,每个分析期间的时间可用于准备以下样本或同时处理另一个系统。这样,吞吐量就会增强,并且每次分析的成本降低。
概括
在许多研究中,自动滴定对手动程序的好处是详细说明的。除了生产率,准确性和准确性的显着提高,人类对分析的影响也降低了。
这些点结合起来,与手动滴定相比,与手动滴定相比,自动滴定更易于使用,除了结果更具可比性和可重现的结果。
References and Further Reading
- Szabadváry,F。History of Analytical Chemistry,,,,1st edition; Belcher, R., Gordon, L. Eds.; Pergamon, 1966.
- Becker,P。历史和准确确定Avogadro常数的进展。Rep. Prog. Phys.2001,,,,64,,,,1945–2008.
- Achinstein,P。Evidence, Explanation, & Realism; Oxford University Press, 2010.
- Jander,G。;Jahr,K。F.Massanalyse,第15版;Schulze,G.,Simon,J。Eds。德·格鲁特(De Gruyter):柏林,纽约,1989年。

此信息已从Metrohm AG提供的材料中采购,审查和调整。亚博网站下载
For more information on this source, please visitMetrohm Ag。